The dental news portal zm-online and the website for medical news DocCheck recently published a statement at the same time on the latest studies concerning vitamin D in oncology (1,2). Both websites refer to a team of researchers from Cologne, who identified a relationship between vitamin D deficiency and survival rate in patients with Hodgkin’s lymphoma (3).
Despite the available scientific data and increasing evidence for the outstanding importance of vitamin D, regular vitamin D determination is not recommended in current guidelines. Furthermore, there is no recommendation for a vitamin D supplementation. This is based on the fact that the actual benefit of administering vitamin D to patients has not yet been verified. It is suggested to implement further studies to prove the positive influence of vitamin D on the survival rate of cancer patients. In case of positive results, the determination and supplementation of vitamin D and other vitamins can be included in the oncological routine (1).
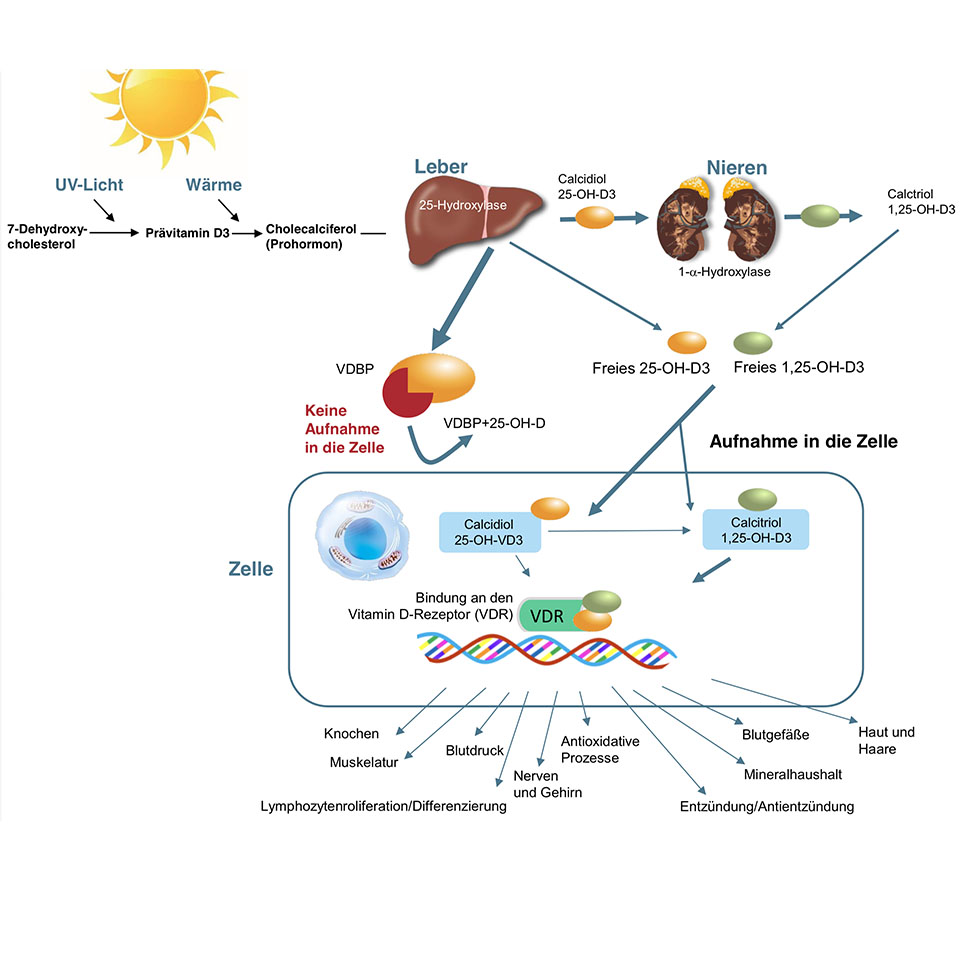
Vitamin D is more than just a vitamin; it is the precursor of a fat-soluble hormone that is required by almost every cell in the body for its physiological function (4–6). The production of the active 1,25-dihydroxy vitamin D, also known as calcitriol, requires several steps. In the skin, under the influence of sunlight and heat, the cholesterol derivative (7-dehydroxy-cholesterol) is converted via the precursor previtamin D3 into the prohormone (cholecalciferol). Further steps are the conversion into calcidiol (25-OH-D3) in the liver to active calcitriol (1,25-OH-D3) in the kidneys. The conversion into the active vitamin D3 is regulated hormonally and depends on the calcium concentration in the blood.
Calcitriol enters the cell and binds to the vitamin D receptor. This receptor is found in nearly all cells of the body.
Vitamin D3 is responsible for the regulation of calcium intake, maintenance of an adequate calcium level in the blood and bone metabolism. Moreover, it has many other functions and controls more than 2.000 genes (6,7).
For adequate functioning vitamin D requires further cofactors such as magnesium, vitamin K2 and zinc (7). Magnesium is essential for the enzymatic conversion into active vitamin D3 (8). Vitamin K2 is crucial for utilization of calcium and prevents the development of hypercalcemia or arteriosclerosis (9–11). Further interesting aspects about vitamin K2 can be read in the newsletter of Dr. Ulrich Volz: VITAMIN K2 / MK7 – ANOTHER SUPERHERO VITAMIN? ( https://www.swiss-biohealth.com/en/vitamin-k2-mk7-en ). Zinc is required because the vitamin D receptor consists of two zinc molecules at its base (7,12).
The functions of vitamin D are immensely important. Vitamin D receptors are present in almost every cell of the body which indicates that it is needed ubiquitously.
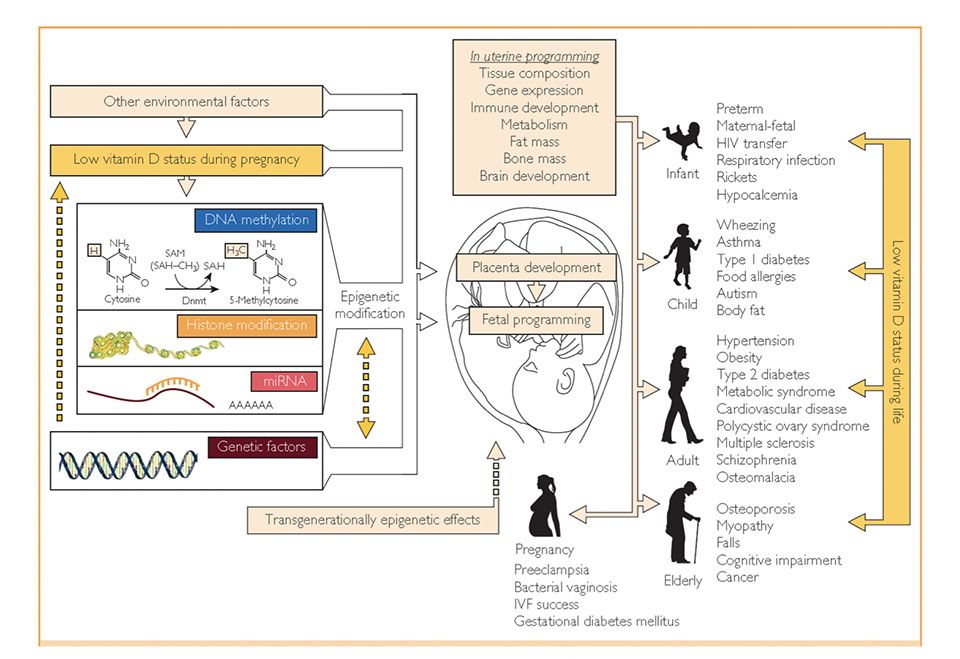
The following figure illustrates that an adequate vitamin D level is essential for all ages, from fetus to elderly people:
Vitamin D3 plays a key role in the mineral and bone homeostasis (14). Moreover, current study results confirm the positive effects of vitamin D in the following five functional areas:
– Fertility/pregnancy (15,16)
– Cardiovascular disease, diabetes and metabolic syndrome (17–20)
– Immune system (21,22)
– Nervous system (23,24)
– Cancer (25)
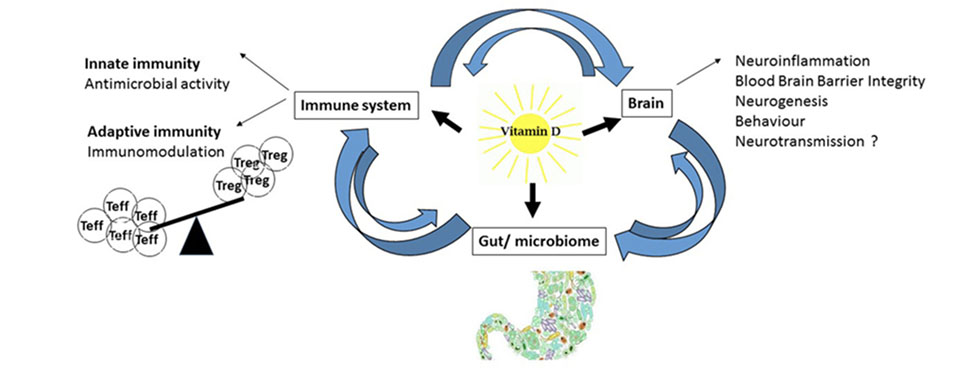
The following facts are available: Pregnant women are recommended to achieve vitamin D levels above 40ng/ml (25-OH-D3) to protect mother and fetus (26). Vitamin D deficiency is associated with increases in the prevalence of diabetes, hypertension and might play a primary role as a cardiovascular risk factor (18). Vitamin D modulates the immune system and protects against (viral) infections as well as autoimmune diseases such as multiple sclerosis (27–29). Neurological diseases such as schizophrenia or autism are vitamin D-dependent, even the microbiome, our largest immune organ, is dependent on vitamin D (30).
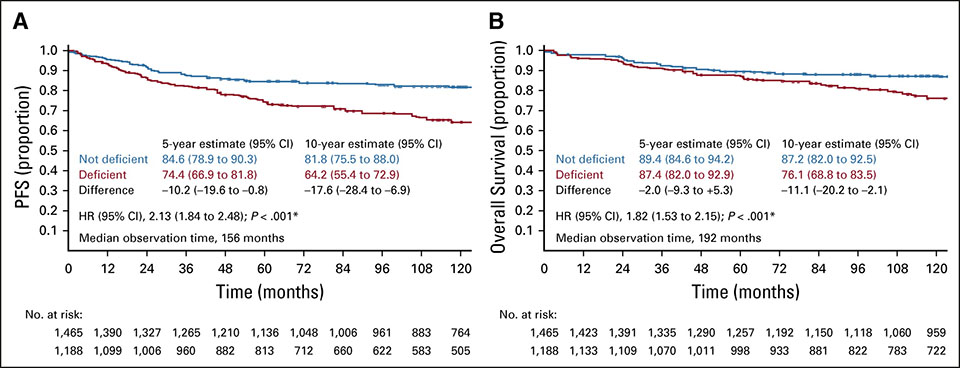
In current oncology studies many investigate the effects of Vitamin D. They show that vitamin D insufficiency in patients with colon cancer, breast cancer, chronic lymphocytic leukemia and acute myeloid leukemia is linked to poorer clinical outcome and prognosis (31–33). Another study stated that patients, diagnosed with B-cell lymphoma, benefit from vitamin D because it enhances rituximab efficacy during chemotherapy (34). Among patients with metastatic colorectal cancer, high-dose vitamin D resulted in a higher progression-free survival (35). The initially mentioned study of the working group of Dr. Sven Borchmann in Cologne evaluated if vitamin D deficiency is a risk factor resulting in poorer tumor control (in 351 Hodgkin’s lymphoma patients over an observation period of 13 years). They were able to prove that patients with vitamin D deficiency – resulting from poorer tumor control – had impaired progression-free survival and overall survival (3).
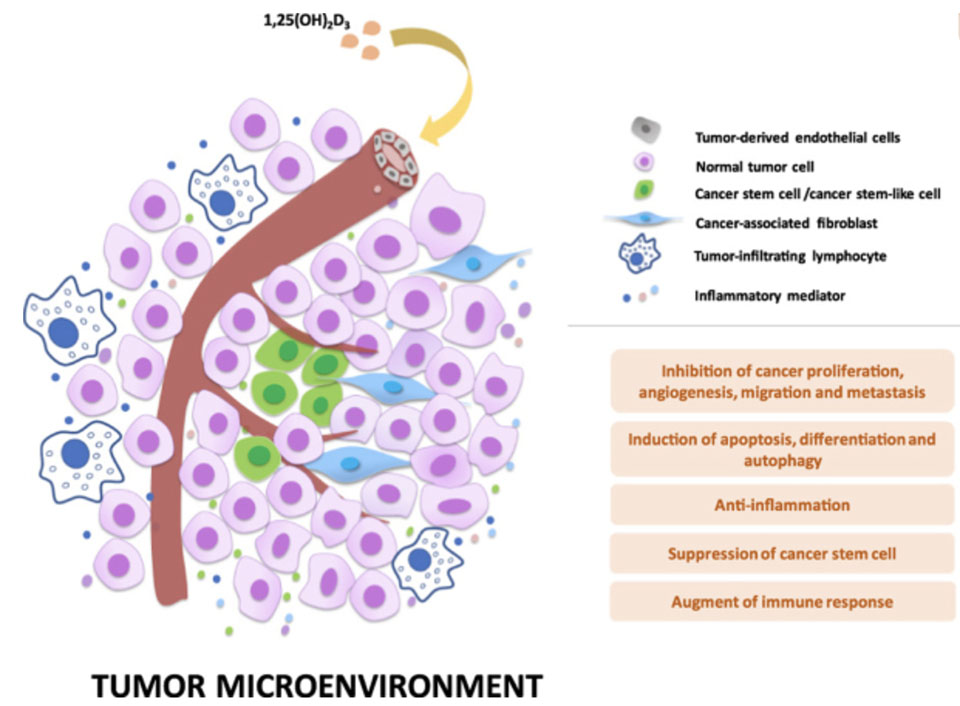
Wu et al. summarized that vitamin D showed anticancer action and proposed vitamin D to be a novel and economical anticancer agent (36).
Unfortunately, vitamin D deficiency occurs all over the world (37).
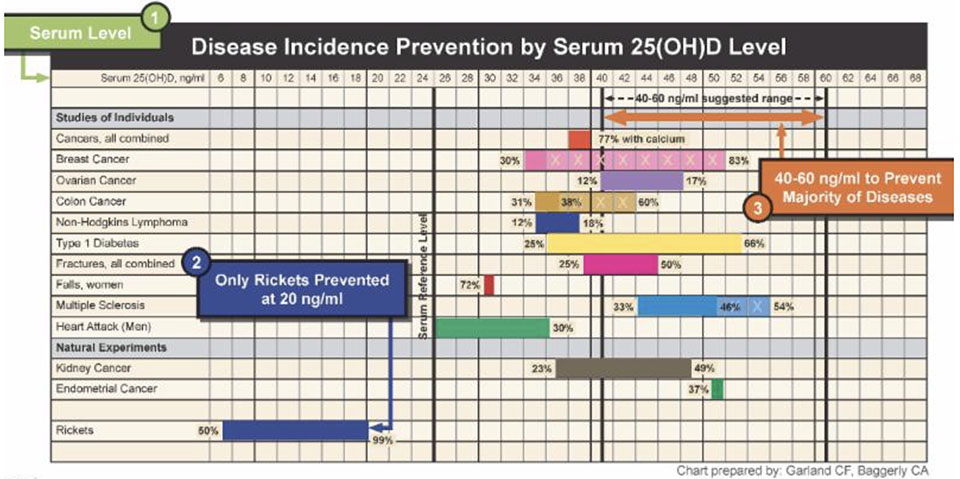
The following figure demonstrates that patients with a vitamin D level of 40 – 60ng/ml are protected against most chronic diseases:
Vitamin D in dentistry
Positive effects of vitamin D can be recognized in almost every medical field. The importance of vitamin D has been reported several times in dentistry. Thus, correlations were documented between vitamin D and caries, molar incisor hypomineralisation and gingivitis/periodontitis (39–44). Vitamin D inhibits the growth and virulence factor gene expression of the periodontal pathogenic bacterium Porphyromonas gingivalis (45); furthermore, vitamin D induced an increase in antibacterial activity against the periodontal pathogen Aggregatibacter actinomycetem-comitans (46). The local bone remodeling might be better when serum vitamin D levels were improved (47).
On the other hand, vitamin D deficiency slows the implant osseointegration and increases the risk of graft infection (48–50).
Summary
It is evident (according to the mentioned studies) that an adequate vitamin D level is of great importance for the human organism.
Further randomized clinical studies on oncological diseases are required to verify the benefit of a vitamin D supplementation and to integrate it into the standard oncological treatment.
The Swiss Biohealth Clinic has established already vitamin D supplementation for every patient to optimize patient’s health and to improve postoperative wound and bone healing. Prior to surgery, a vitamin D level of at least 70 ng/ml is targeted.
Author: Dr. Stephanie Vergote
Member of Swiss Biohealth Academy
References
1. Doccheck. Vitamin D bei Tumoren: Die drei ??? [Internet]. 2019. Available from: https://www.doccheck.com/de/detail/articles/23723-vitamin-d-bei-tumoren-die-drei?utm_source=DC-Newsletter&utm_medium=email&utm_campaign=Newsletter-DE-DocCheck%20News%2019.45%20(Donnerstag)-2019-11-07&utm_content=asset&utm_term=article
2. zm-online. Hodgkin Lymphom: Vitamin-D-Mangel verschlechtert Überleben [Internet]. 2019. Available from: https://www.zm-online.de/news/gesellschaft/hodgkin-lymphom-vitamin-d-mangel-verschlechtert-ueberleben
3. Borchmann S, Cirillo M, Goergen H, Meder L, Sasse S, Kreissl S, Bröckelmann PJ, Tresckow B v., Fuchs M, Ullrich RT, Engert A. Pretreatment Vitamin D Deficiency Is Associated With Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. Journal of Clinical Oncology. 2019;JCO.19.00985. doi:10.1200/JCO.19.00985
4. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S-96S. doi:10.1093/ajcn/80.6.1689S
5. Bikle D. Nonclassic Actions of Vitamin D. J Clin Endocrinol Metab. 2008;94(1):26–34. doi:10.1210/jc.2008-1454
6. Zittermann A, Gummert JF. Nonclassical vitamin D action. Nutrients. 2010;2(4):408–25. doi:10.3390/nu2040408
7. Schweihart Vitamin D – das Sonnenhormon. Vitamin D Stoffwechsel [Internet]. Available from: https://www.vitamind.net/vitamin-d3/stoffwechsel/
8. Zittermann A. Magnesium deficit ? overlooked cause of low vitamin D status? BMC Med. 2013;11229. doi:10.1186/1741-7015-11-229
9. Masterjohn C. Vitamin D toxicity redefined: vitamin K and the molecular mechanism. Med Hypotheses. 2007;68(5):1026–34. doi:10.1016/j.mehy.2006.09.051
10. Schurgers LJ, Cranenburg ECM, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100(4):593–603.
11. Flore R, Ponziani FR, Di Rienzo TA, Zocco MA, Flex A, Gerardino L, Lupascu A, Santoro L, Santoliquido A, Di Stasio E, Chierici E, Lanti A, Tondi P, Gasbarrini A. Something more to say about calcium homeostasis: the role of vitamin K2 in vascular calcification and osteoporosis. Eur Rev Med Pharmacol Sci. 2013;17(18):2433–40.
12. Freedman LP, Towers TL. DNA Binding Properties of the Vitamin D3 Receptor Zinc Finger Region: Cell Biology and Genetics Program Sloan-Kettering Institute New York, New York 10021. Molecular Endocrinology. 1991.
13. Holick. Vitamin D, placenta development, fetal programming, and epigenetic modification. A— adenosine; CH3 — methylgroup; HIV — human immunodeficiency virus; IVF — in vitro fertilization; miRNA, micro RNA; SAH — S-adenosylhomocysteine; SAM— single carbon from adenosylmethionine [Internet]. 2013. Available from: https://vitamindwiki.com/Vitamin+D+Global+Perspective+-+Holick+June+2013
14. Sawatsubashi S. Bone and Nutrition. The vitamin D functions in osteoblasts and osteocytes. Clin Calcium. 2015;25(7):991–7. jpn.
15. Hollis BW, Wagner CL. Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Mol Cell Endocrinol. 2017;453113–30. doi:10.1016/j.mce.2017.01.039
16. Voulgaris N, Papanastasiou L, Piaditis G, Angelousi A, Kaltsas G, Mastorakos G, Kassi E. Vitamin D and aspects of female fertility. Hormones (Athens). 2017;16(1):5–21. doi:10.14310/horm.2002.1715
17. Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, Kavtaradze N, Uphoff I, Hooper C, Tangpricha V, Alexander RW, Brigham K, Quyyumi AA. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. J Am Coll Cardiol. 2011;58(2):186–92. doi:10.1016/j.jacc.2011.02.051
18. Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, Lappé DL, Muhlestein JB. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106(7):963–8. doi:10.1016/j.amjcard.2010.05.027
19. Dimova R, Tankova T, Chakarova N. Vitamin D in the Spectrum of Prediabetes and Cardiovascular Autonomic Dysfunction. J Nutr. 2017;147(9):1607–15. doi:10.3945/jn.117.250209
20. Szep Z, Guaraldi G, Shah SS, Lo Re V, Ratcliffe SJ, Orlando G, Carli F, Rossi R, Rochira V, Tebas P. Vitamin D deficiency is associated with type 2 diabetes mellitus in HIV infection. AIDS. 2011;25(4):525–9. doi:10.1097/QAD.0b013e328342fdfd
21. Kamen DL, Tangpricha V. Vitamin D and molecular actions on the immune system: modulation of innate and autoimmunity. J Mol Med. 2010;88(5):441–50. doi:10.1007/s00109-010-0590-9
22. Cantorna MT, Zhao J, Yang L. Vitamin D, invariant natural killer T-cells and experimental autoimmune disease. Proc Nutr Soc. 2012;71(1):62–6. doi:10.1017/S0029665111003193
23. Wrzosek M, Łukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piątkiewicz P, Radziwoń-Zaleska M, Wojnar M, Nowicka G. Vitamin D and the central nervous system. Pharmacol Rep. 2013;65(2):271–8. doi:10.1016/s1734-1140(13)71003-x
24. Holmøy T, Moen SM. Assessing vitamin D in the central nervous system. Acta Neurol Scand , Suppl. 2010;(190):88–92. doi:10.1111/j.1600-0404.2010.01383.x
25. Holick MF. Vitamin D: A millenium perspective. J Cell Biochem. 2003;88(2):296–307. doi:10.1002/jcb.10338
26. Wagner CL, Hollis BW. The Implications of Vitamin D Status During Pregnancy on Mother and her Developing Child. Front Endocrinol (Lausanne). 2018;9500. doi:10.3389/fendo.2018.00500
27. Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151–65. doi:10.2217/fmb.09.87
28. Berry DJ, Hesketh K, Power C, Hyppönen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106(9):1433–40. doi:10.1017/S0007114511001991
29. Cantorna MT, Mahon BD. Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood). 2004;229(11):1136–42. doi:10.1177/153537020422901108
30. Kočovská E, Gaughran F, Krivoy A, Meier U-C. Vitamin-D Deficiency As a Potential Environmental Risk Factor in Multiple Sclerosis, Schizophrenia, and Autism. Front Psychiatry. 2017;847. doi:10.3389/fpsyt.2017.00047
31. Maalmi H, Ordóñez-Mena JM, Schöttker B, Brenner H. Serum 25-hydroxyvitamin D levels and survival in colorectal and breast cancer patients: systematic review and meta-analysis of prospective cohort studies. Eur J Cancer. 2014;50(8):1510–21. doi:10.1016/j.ejca.2014.02.006
32. Shanafelt TD, Drake MT, Maurer MJ, Allmer C, Rabe KG, Slager SL, Weiner GJ, Call TG, Link BK, Zent CS, Kay NE, Hanson CA, Witzig TE, Cerhan JR. Vitamin D insufficiency and prognosis in chronic lymphocytic leukemia. Blood. 2011;117(5):1492–8. doi:10.1182/blood-2010-07-295683
33. Lee HJ, Muindi JR, Tan W, Hu Q, Wang D, Liu S, Wilding GE, Ford LA, Sait SNJ, Block AW, Adjei AA, Barcos M, Griffiths EA, Thompson JE, Wang ES, Johnson CS, Trump DL, Wetzler M. Low 25(OH) vitamin D3 levels are associated with adverse outcome in newly diagnosed, intensively treated adult acute myeloid leukemia. Cancer. 2014;120(4):521–9. doi:10.1002/cncr.28368
34. Bittenbring JT, Neumann F, Altmann B, Achenbach M, Reichrath J, Ziepert M, Geisel J, Regitz E, Held G, Pfreundschuh M. Vitamin D deficiency impairs rituximab-mediated cellular cytotoxicity and outcome of patients with diffuse large B-cell lymphoma treated with but not without rituximab. J Clin Oncol. 2014;32(29):3242–8. doi:10.1200/JCO.2013.53.4537
35. Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, Rubinson DA, Schrag D, Miksad R, Bullock AJ, Allen J, Zuckerman D, Chan E, Chan JA, Wolpin BM, Constantine M, Weckstein DJ, Faggen MA, Thomas CA, Kournioti C, Yuan C, Ganser C, Wilkinson B, Mackintosh C, Zheng H, Hollis BW, Meyerhardt JA, Fuchs CS. Effect of High-Dose vs Standard-Dose Vitamin D3 Supplementation on Progression-Free Survival Among Patients With Advanced or Metastatic Colorectal Cancer: The SUNSHINE Randomized Clinical Trial. JAMA. 2019;321(14):1370–9. doi:10.1001/jama.2019.2402
36. Wu X, Hu W, Lu L, Zhao Y, Zhou Y, Xiao Z, Zhang L, Zhang H, Li X, Li W, Wang S, Cho CH, Shen J, Li M. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B. 2019;9(2):203–19. doi:10.1016/j.apsb.2018.09.002
37. van Schoor N, Lips P. Global Overview of Vitamin D Status. Endocrinol Metab Clin North Am. 2017;46(4):845–70. doi:10.1016/j.ecl.2017.07.002
38. Garland CF BCA. Disease Incidence Prevention by Serum 25(OH)D Level [Internet]. Available from: http://files.ctctcdn.com/25aa06fb001/cc1fc56c-f98d-42b3-98d1-d6e2f1cd4a65.jpg
39. Kim I-J, Lee H-S, Ju H-J, Na J-Y, Oh H-W. A cross-sectional study on the association between vitamin D levels and caries in the permanent dentition of Korean children. BMC Oral Health. 2018;18(1):43. doi:10.1186/s12903-018-0505-7
40. Schroth RJ, Rabbani R, Loewen G, Moffatt ME. Vitamin D and Dental Caries in Children. J Dent Res. 2016;95(2):173–9. doi:10.1177/0022034515616335
41. Kühnisch J, Thiering E, Kratzsch J, Heinrich-Weltzien R, Hickel R, Heinrich J. Elevated serum 25(OH)-vitamin D levels are negatively correlated with molar-incisor hypomineralization. J Dent Res. 2015;94(2):381–7. doi:10.1177/0022034514561657
42. Bhargava A, Rastogi P, Lal N, Singhal R, Khatoon S, Ali Mahdi A. Relationship between VITAMIN D and chronic periodontitis. J Oral Biol Craniofac Res. 2019;9(2):177–9. doi:10.1016/j.jobcr.2018.07.001
43. Meghil MM, Hutchens L, Raed A, Multani NA, Rajendran M, Zhu H, Looney S, Elashiry M, Arce RM, Peacock ME, Dong Y, Cutler CW. The influence of vitamin D supplementation on local and systemic inflammatory markers in periodontitis patients: A pilot study. Oral Dis. 2019;25(5):1403–13. doi:10.1111/odi.13097
44. Nørrisgaard PE, Haubek D, Kühnisch J, Chawes BL, Stokholm J, Bønnelykke K, Bisgaard H. Association of High-Dose Vitamin D Supplementation During Pregnancy With the Risk of Enamel Defects in Offspring: A 6-Year Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2019. doi:10.1001/jamapediatrics.2019.2545
45. Grenier D, Morin M-P, Fournier-Larente J, Chen H. Vitamin D inhibits the growth of and virulence factor gene expression by Porphyromonas gingivalis and blocks activation of the nuclear factor kappa B transcription factor in monocytes. J Periodont Res. 2016;51(3):359–65. doi:10.1111/jre.12315
46. McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect Immun. 2011;79(6):2250–6. doi:10.1128/IAI.00099-11
47. Schulze-Späte U, Dietrich T, Wu C, Wang K, Hasturk H, Dibart S. Systemic vitamin D supplementation and local bone formation after maxillary sinus augmentation – a randomized, double-blind, placebo-controlled clinical investigation. Clin Oral Implants Res. 2016;27(6):701–6. doi:10.1111/clr.12641
48. Choukroun J, Khoury G, Khoury F, Russe P, Testori T, Komiyama Y, Sammartino G, Palacci P, Tunali M, Choukroun E. Two neglected biologic risk factors in bone grafting and implantology: high low-density lipoprotein cholesterol and low serum vitamin D. J Oral Implantol. 2014;40(1):110–4. doi:10.1563/AAID-JOI-D-13-00062
49. Bryce G, MacBeth N. Vitamin D deficiency as a suspected causative factor in the failure of an immediately placed dental implant: a case report. J R Nav Med Serv. 2014;100(3):328–32.
50. Cooper LF. Systemic effectors of alveolar bone mass and implications in dental therapy. Periodontol 2000. 2000;23103–9. doi:10.1034/j.1600-0757.2000.2230110.x